Vaccine Vero Cell - Coronavirus Who Approves Sinovac Covid Vaccine For Emergency Use News Dw 01 06 2021
Sinopharm vaccine Vero cell weak or inactivated SARS-2 Cov-2 vaccine. It is the also first vaccine that will carry a vaccine vial monitor a small sticker on the vaccine vials that change color as the vaccine is exposed to heat letting health workers know whether the vaccine can be safely usedWHOs.
North Korea Rejects Offer Of Nearly 3 Million Sinovac Covid 19 Shots Reuters
SARS-Cov-2 Vaccine Vero Cell Market Analysis In the competitive analysis section of the report leading as well as prominent players of the SARS-Cov-2 Vaccine Vero Cell market are broadly.

Vaccine Vero Cell. Rapid development and production of vaccines against emerging diseases requires well established validated robust technologies to allow industrial scale. SARS-CoV-2 Vaccine Vero Cell Inactivated Coronavac - Food and Drug Administration SARS-CoV-2 Vaccine Vero Cell Inactivated Coronavac On 22 February 2021 the Food and Drug Administration FDA issued authorization granting IP BIOTECH INC. The Vero lineage was isolated from kidney epithelial cells extracted from an African green monkey.
Formalin inactivated vaccine elicited high virus specific IgG antibody titer and neutralization titer. Inactivated SARS-CoV-2 Virus CZ02 s train Adjuvant. And neutralizing antibody on a standard 50 microneutralization assay in vero cells.
Vero cell vaccine sinopharm or sinovac. Sinopharm have manufactured two vaccines developed with the Institute of Biological Products in Beijing and Wuhan respectively. Từ ngày 148 TPHCM bắt đầu triển khai tiêm vaccine Vero Cell của hãng Sinopharm trên diện diện rộng.
Efficacy Safety and Immunogenicity of Inactivated SARS-CoV-2 Vaccines Vero Cell to Prevent COVID-19 in Healthy Adult Population In Peru Healthy Adult Population In Peru Covid-Peru The safety and scientific validity of this study is the responsibility of the study sponsor and investigators. Vero Cell Vaccine Efficacy. Its easy storage requirements make it highly suitable for low-resource settings.
Disodium hydrogen phosphate dodecahydra te sodium dihydrogen phosphate monohydrate sodium chloride DESCRIPTION CoronaVac is a milky-white suspension. The primary objective is to evaluate the efficacy of an inactivated and aluminium hydroxide adsorbed. The emergency use approval of SARS-CoV-2 Vaccine Vero Cell Inactivated Coronavac.
According to the decision the Vero Cell vaccine will be administered to migrant workers alongside people above 55 years staff members of schools and colleges transport workers and those working in sectors defined as essential services by the government. If later the vaccinated person comes into contact with SARS-CoV-2 the immune system will recognise the virus and be. Vero cells are a lineage of cells used in cell cultures.
The original cell line was named Vero after an abbreviation of verda reno which means green kidney in Esperanto while vero itself. When a person is given the vaccine their immune system identifies the inactivated virus as foreign and makes antibodies against it. 30 sinopharm announces that the vaccine has an efficacy of 7934 percent leading the chinese government to approve itthe company.
Avidity of the IgG antibodies elicited by two different doses of formalin inactivated vaccine is moderately high which got augmented by alum adjuvanted formulations. COVID-19 Vaccine Vero Cell Inactivated COMPOSITION Active ingredient. Referring to the above principles the SARS-CoV-2 inactivated vaccine Vero cells was administered once each on days 0 14 28 and 42 for a total of four administrations and the interval was 2 weeks followed by a recovery period after the last administration of 2 weeks 14 days.
The COVID-19 Vaccine Vero Cell Inactivated CoronaVac is an inactivated vaccine against coronavirus disease 2019 COVID-19 which stimulates the bodys immune system without risk of causing a disease. The COVID-19 vaccine under. KAZINFORM Another batch of Chinas Vero Cell vaccine arrived in Kazakhstan Healthcare Minister Alexey Tsoi said.
The Sinopharm product is an inactivated vaccine called SARS-CoV-2 Vaccine Vero Cell. Sinovacs Coronavac SARS-CoV-2 Vaccine Vero Cell Inactivated Announces Approval for Phase III Clinical Trial in Adolescents and Children. Formalin inactivated unadjuvanted vaccine elicited a balanced IgG1.
The Vero cell adapted virus was inactivated with Formalin and immunized in mice. COVID-19 Vaccine Vero Cell Inactivated also contains an adjuvant a substance that helps strengthen the immune response to the vaccine. A Study to Evaluate The Efficacy Safety and Immunogenicity of Inactivated SARS-CoV-2 Vaccines Vero Cell in Healthy Population Aged 18 Years Old and Above COVID-19 The safety and scientific validity of this study is the responsibility of the study sponsor and investigators.
The COVID-19 Vaccine BIBP also known as Inactivated COVID-19 VERO CELL vaccine is produced in Vero Cells and inactivated with ß-propiolactone. The Sinopharm COVID-19 vaccine vero cell inactivated vaccine resource includes key information on the vaccine specific requirements. HCM CITY A total of 85608 people in HCM City were vaccinated with Sinopharms COVID-19 vaccine Vero Cell on Saturday the municipal Department of Health announced on Sunday.
Vero cell technology for rapid development of inactivated whole virus vaccines for emerging viral diseases. China donated Covid-19 vaccine Vero Cell arrives in Kathmandu According to the Health ministry these jabs will be received by those involved in operation of. The lineage was developed on 27 March 1962 by Yasumura and Kawakita at the Chiba University in Chiba Japan.

Coronavirus Who Approves Sinovac Covid Vaccine For Emergency Use News Dw 01 06 2021

Who Approves A Covid 19 Vaccine From China S Sinopharm For Emergency Use
Sinovac Covid 19 Vaccine Safe For Ages 3 To 17 In Small Early Trial Devex
Singapore Excludes Sinovac Shots From Covid 19 Vaccination Tally Nikkei Asia
Sinopharm Vaccine Side Effects Price Efficacy Of China S Sinopharm Covid Vaccine India News Times Of India
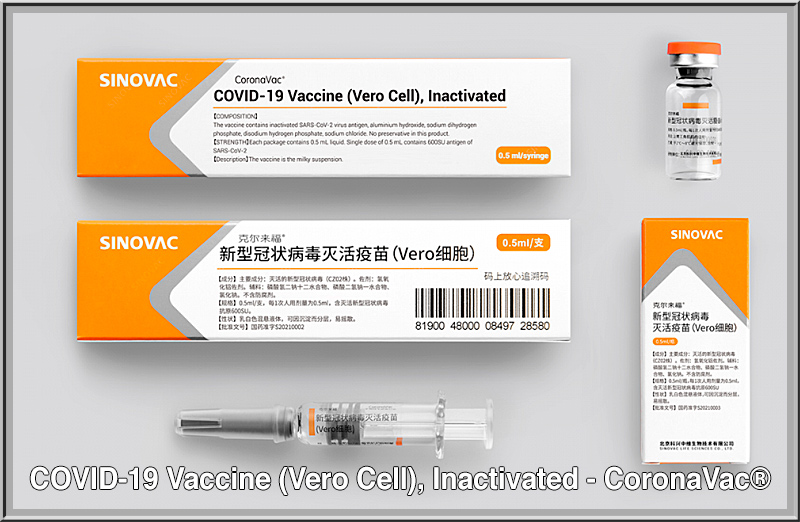
Nieuws Ema Test Het Covid 19 Vaccine Vero Cells Van China

Covid 19 Chinese Official Says Homegrown Vaccines Not Very Powerful Euronews

European Drugs Regulator Starts Rolling Review Of Sinovac Anti Coronavirus Jab

China Vaccine Vero Cell Green Light For Human Trial In Bangladesh The Daily Star

Who Approves China S Sinopharm Covid 19 Vaccine For Emergency Use Has 79 Efficacy Coronavirus Outbreak News

Who Lists Sinopharm S Covid 19 Vaccine For Emergency Use

Sinopharm Vero Cell Inactivated Covid 19 Vaccine

Eu Regulator Begins Real Time Review Of First Chinese Covid 19 Vaccine Reuters

China S Covid Vaccine From Sinopharm Is 86 Effective Uae Says
China S Sinopharm Vaccine Receives 1st Eu Gmp Certificate Cgtn

Chinese Vaccines Show Promise As Booster Shots As More Study Results Shared 2021 09 08 Bioworld

.JPG)